8.1 Introduction
Diabetic maculopathy (DM) causes gradual and largely irreversible loss
of central vision. many patients with DM are elderly; thus assessment
and treatment may be complicated by co-existing ocular pathology,
such as cataract, glaucoma or a poor mydriasis response. Medico-social
problems may also militate against optimal management.
.
Diabetic maculopathy is caused by oedema from leaking capillaries
and/or ischaemia due to capillary loss. Diabetic changes to the choroidal
vasculature may also worsen the ischaemia and contribute to the
oedema by reducing retinal pigment epithelial function. Preventable
visual loss is largely due to disruption of the fovea and perifoveal neuro-
retina by oedema. The ETDRS and the British Multi-centre Photo-
coagulation Study showed that appropriate macular photocoagulation
was effective in preventing central visual loss due to macular oedema,
in many cases, for up to seven years. Optimal management depends on
detection and treatment by photocoagulation of oedema before the
fovea is involved. The aim of treatment is to prevent further visual loss,
since visual acuity rarely improves following treatment, best results are
achieved by applying treatment before central vision deteriorates.
.
Loss of central vision due to macular ischaemia or generalized oedema
cannot be prevented by photocoagulation. In young people visual acuity
may be preserved despite macular ischaemia. Many cases of diabetic
maculopathy involved co-existing macular oedema and ischaemia. In
such cases, the prognosis depends on the extent of the oedema and
the severity of the ischaemia. Central vision can remain surprisingly good,
even with marked retinal capillary closure, provided coexisting oedema is
treated before it involves the fovea.
.
8.2 Indications for treatment
Treatment is indicated when the maculopathy threatens the fovea or
perifoveal area. Isolated microaneurysms around the fovea without
clinical evidence of retinal thickening therefore do not merit treatment,
but they should continue to be observed at regular intervals. Small
localized areas of retinal thickening outside the central macula area
do not require treatment, though such cases do require regular follow-up.
Fluorescein angiographic evidence of retinal oedema in the absence of
clinically obvious retinal thickening (Clinically Significant Macular Oedema,
CSME in the ETDRS) is not normally regarded as an indication for
treatment. .
8.3 Treatment protocols for diabetic maculopathy
The aim of treatment is resolution of retinal oedema before the fovea is
involved. This is achieved using laser therapy to the macula. How laser
therapy achieves this effect is unclear. There is either a direct effect on
leaking microvascular complexes in the retinal circulation or an indirect
effect mediated through the retinal pigment epithelium.
.
Safe treatment depends on accurate identification of the fovea and
avoidance of excessively intense burns. The fovea can be difficult to
identify if there is considerable oedema. Excessively intense burns can
be avoided by starting treatment with very low powered burns, and
gradually increasing the intensity until a satisfactory moderate blanching
of the retina is achieved. Energy uptake varies, depending on the degree
of the retinal oedema, so it is important to reduce power when treating
less oedematous areas.
.
Satisfactory results can be achieved with a number of different light
wavelengths (see elsewhere in these guidelines). The most frequently
used wavelengths are 514nm (the ‘Green’ component of the Argon
Blue/Green laser) and 810nm (from the infra-red diode laser). The Argon
blue/green laser should not be used for treatment of microaneurysms
that are very close to the central area. This is because the blue light
from this laser (487nm) is absorbed by xanthophylls pigment overlying
the parafoveal area. this can cause nerve fibre layer damage and
parafoveal scotomata (see section on lasers).
In general, the blue laser waveband is not recommended due to its
possible deleterious effects on the user (see Section 5.2).
.
8.3.1 Focal maculopathy
This type of maculopathy responds most readily to photocoagulation.
Areas of focal leakage, usually a the centre of the exudative rings
(identifiable if necessary by fluorescein angiography but only if doubt
exists as to their precise location), are treated using a 50-100 micron
beam at sufficient power level to obtain moderate blanching of the
retina. When the microaneurysm is close to the fovea, a short
superficial burn just sufficient to blanch it may be used.
However, the treatment of microaneurysms within 300 micron of the centre
of the fovea should be undertaken with caution because of the significant
risk of closure of the perifoveal arcade.
An alternative to direct treatment of focal leakage is to apply a gentle grid
to the entire circinate ring, including the margin, in a fashion similar to that
used for diffuse maculopathy.
.
.
8.3.2 Diffuse maculopathy
Diffuse maculopathy is a more difficult form of diabetic retinopathy to treat.
However, grid laser photocoagulation offers the only available, validated
therapy. The technique consists of applying 100-200 micron burns delivered
at a power level sufficient to obtain a minimum blanching reaction the
pigment epithelium in a grid pattern over the central macula avoiding the
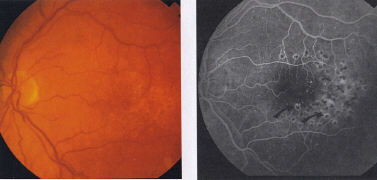
fovea itself (Figure 26a, b). Patients may visualize the grid entoptically. .
8.3.3. Mixed maculopathy
Focal and diffuse areas of oedema should be identified and treated as above. .
8.3.4 ischaemic maculopathy
Ischaemic maculopathy does not respond to laser therapy. However, if the
degree of ischaemia is sufficiently large that it performs part of the pre-
proliferative retinopathy, then this of itself may warrant therapy. .
8.3.5 Diffuse non-responsive macular oedema
Severe diffuse macula redeem which is non-responsive to grid laser
photocoagulation or repeated grid laser photocoagulation, may benefit from
vitrectomy with removal of the attached posterior hyaloid face. Cases likely
to benefit have an appearance which may be very subtle, but presents as
posterior hyoid face thickening, surface wrinkling and a detectable sheen, or
abnormal reflex from the inner limiting lamina. The surgical goal should be the
removal of all thickened posterior hyaloid and cortical gel material via a three
port pars plana vitrectomy (see next section). Approximately 50% of patients
may experience moderate improvement of vision, up to 2 lines or more.
.
8.3.6 Diffuse maculopathy in the presence of neovascularization
Occasionally maculopathy co-exists with disc or retinal neovascularization.
Whether to treatment the new vessels with PRP or to treat the
maculopathy first depends on the age of the patient and the relative
severity of the retinopathy. In young patients with active new vessels it
is generally recommended to treat the new vessels first with PRP since new
vessels in these patients can advance rapidly with devastating consequences.
In older patients with NIDDM, it is better to treat the maculopathy first or at
least at the same time since PRP itself in these patients can hasten the
progression of the maculopathy. Fractionating PRP into sessions of 700-800
burns separated by 2-3 weeks reduces the risk of this progression.
. 8.4 Follow-up after treatment of diabetic maculopathy
Patients with diabetic maculopathy should be reviewed 3-4 months after
treatment. If oedema persists, or if the visual acuity worsens, repeat
fluorescein angiography may be helpful in identifying areas of residual
focal leakage which should then be retreated. Treatment of leaking areas
associated with exudates may cause regression of the exudates over
many months.
Since focal maculopathy can recur, patients with treated maculopathy
should be followed for a sufficient time to ensure that the condition is
inactive. Patients with cataracts and treated diabetic retinopathy should
have the maculopathy reassessed before cataract surgery since surgery
can induce recurrence or advance of existing macular oedema. Any persistent
areas of oedema should be re-treated either before or soon after surgery.
..............