
 75th Anniversary of the Discovery
of Insulin.(1921-1996) Banting
and Best. Extraction from the islets
which they called Isletin (insulin)
control the blood glucose of dogs
rendered diabetic through removal
of their pancreases. The first person
to receive Isletin was a 14 year old
boy Leonard Thompson with IDDM.
He lived for 13 years and died from
severe diabetic ketoacidosis.
75th Anniversary of the Discovery
of Insulin.(1921-1996) Banting
and Best. Extraction from the islets
which they called Isletin (insulin)
control the blood glucose of dogs
rendered diabetic through removal
of their pancreases. The first person
to receive Isletin was a 14 year old
boy Leonard Thompson with IDDM.
He lived for 13 years and died from
severe diabetic ketoacidosis.



(NVD)
(for a larger view of the retina, click the picture for a popu-up)
The use of lasers to treat diabetic retinopathy is an invasive and destructive
procedure and it is essential to obtain informed consent for each new
treatment course as for any surgical procedure.
.
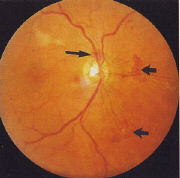
6.1 Definition
NVD are defined as any abnormal collection of leaking vessels occurring on
the optic disc or within one disc diameter of the optic disc. They may be
distinguished from collateral vessels by the presence of dye leakage on
fluorescent angiography.
.
6.2 Treatment
Treatment of NVD (and NVE, see section 7) is by pan-retinal laser photo-
coagulation (PRP). The risk of severe visual loss in high risk patients is
reduced by 50% at 2 and 5 years by this therapy and by up to 70% in
moderate risk patients. PRP should be given with fully informed consent
(see below under counselling) and can be tailored to the nature fo the
NVD in order to minimize effects on the visual field.
.
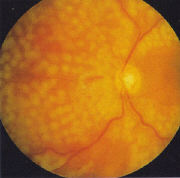
6.2.1 Early NVD
Newly developing flat ‘early’ new vessel usually respond well to a basic
PRP comprising 15000-2000, 200-500 micron laser burns applied pre- and
post-equatorially outside the vascular arcades (Figure 23a, b). Lesions
should normally be applied leaving lesion-wide (ie 200-500 micron)
intervals throughout the fundus to preserve visual field. Some practitioners
prefer routinely to use a smaller spot size such as 200 micron but a
correspondingly larger number of burns is required to achieve an effect.
In addition, spot size will vary with the lens used (see above). Sufficient
energy should be applied to achieve ‘blanching’ (ie. a greyish white lesion)
of the retina without producing visible necrosis. The amount of energy
required will vary for each patient and also at different retinal locations
in the same eye depending on factors such as retinal thickness, oedema
and degree of melanisation of the retinal pigment epithelium (the main
energy absorbing tissue in this procedure).
.
 |
 |
6.2.2 Established NVD
‘ Established’ new vessels are those which have developed into mature
branching and/or arcade formations, but are still ‘flat’ ie they lie on the
retinal surface, and have not led to haemorrhage. Established NVD may
require a full PRP (>2000 laser burns) applied over more than one session.
The precise number of burns will depend on the response to treatment
which should be monitored at 2-3 weekly intervals.
.
6.2.3 Florid NVD
NVD in young adolescents may progress rapidly to extensive sheets of
vessels occupying a wide region of the peripapillary retina (Figure 15).
Such vessels require urgent, aggressive management which should
comprise a full PRP applied in a single session if possible. Laser lesions
should be appropriately ‘ heavy ‘ in florid retinopathy.
Further laser should be applied at weekly intervals until regression of
vessels is achieved. If the neovascularization cannot be controlled,
vitrectomy with endolaser may be required (see below).
.
6.2.4 Stable NVD
NVD respond to PROP either by regressing completely (especially in
older subjects and if treated early) or by ‘maturing’ into non-leaking
smaller vascular formations which do not progress. These can be
described as ‘ stable’ NVD which require observation and monitoring,
if necessary with fluorescent angiography, but probably do not require
further photocoagulation.
.
6.2.5 Non-responding NVD
In a proportion of patients, NVD fail to regress and/or mature into an
inactive stable state. Inadequate laser therapy is a frequent cause,
often due to poor patient compliance with the PRP procedure. This
may be improved by performing the laser with local (retrobulbar) or
general anaesthetic. In the latter case, the laser may be applied via
the indirect ophthalmoscope with good effect and a larger burn size.
Older photocoagulation systems such as Xenon arc may also be useful
in treating wider confluent areas of retina. Even if fully treed as
described above, certain patients still fail to respond. Re-treating
treated areas of the retina may then be performed, usually however
at the expense of the visual field. This will affect the patient’s
fitness to drive and appropriate counselling is essential. Recourse to
vitrectomy may be necessary if the neovascularization cannot be
controlled (see below).
.
6.2.6 Forward NVD
Patient may present with untreated ‘forward’ NVD or the NVD may
project into the vitreous cavity during the course of treatment as a
result of posterior vitreous detachment (PVD). In the absence of
vitreous haemorrhage forward NVD should be treated urgently with a
full PRP with the proviso that an overly aggressive PRP may induce
too rapid a regression of he forward vessels and cause vitreous
bleeding. In such circumstances vitrectomy and intra-operative
endolaser therapy is likely to be the treatment of choice (see below).
.
6.2.7 NVD with vitreous haemorrhage
Treatment of NVD in the presence of small collection of subhyaloid
or intra-gel blood should be aimed at performing a basic PRP (2000
laser burns, 500 micron) followed by careful application of further
brief laser applications (200-300 laser burns per session) until
regression of the NVD is achieved. However, this may not be
successful and vitrectomy with endolaser may be required.
.......