|
| January
2004, Volume 18, Number 1, Pages 98-101 |
 |
| Letter
to the Journal |
| Dermatochalasis: a potential pitfall in
botulinum rejuvenation |
 |
| C N Chua1, A R Gibson1 and N J Rowson2 |
 |
| 1The Eye Unit, Radcliffe Infirmary, Oxford,
OX2 6HE, UK
2The Eye Unit Pembury Hospital Pembury,
Kent, UK |
| Correspondence to: CN Chua, Tel: +44 7770 5043 14;
E-mail: chuaoxford@hotmail.com |
 |
|
Eye (2004) 18, 98-101.
doi:10.1038/sj.eye.6700527
|
 |
 |
| Sir, |
 |
| Since the first publication of the cosmetic use of botulinum toxins
by Carruthers and Carruthers, the use of botulinum toxin has gained popularity
as a safe and reversible method of achieving periorbital rejuvenation.1
While ptosis and subcutaneous haematoma are recognised as complications
other unwanted effects of treatment can occur.2 This letter
describes two cases where botulinum toxin treatment unmasked latent dermatochalasis.
The proposed mechanism of this unwanted effect is discussed and the steps
to be taken to recognise those at risk of developing the complication are
offered. |
 |
| Case 1
A 48-year-old woman complained of sensation of heaviness of the eyelids
and distortion of the upper lids 1 week following botulinum toxin injection
to the upper forehead. A total of 15 U of Botox (Allergen) were diffusely
injected into the forehead wrinkles. Examination revealed a smooth forehead
with absent frontalis action. The eyebrows were in normal position and
lid closure was unaffected. However above both skin creases, there were
redundant folds of skin overhanging the skin creases (Figure
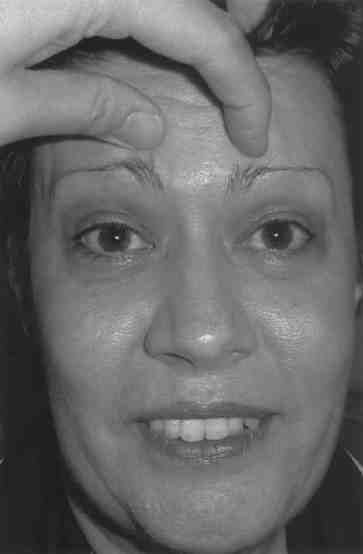
1). Manual elevation of the eyebrows caused the skin to disappear (Figure
2). Reviewing of the photographs taken before the treatment showed
bilateral high eyebrows with apparent frontalis overaction. Paralysis of
the frontalis led to unmasking of latent dermatochalasis. The patient was
given the choice of upper lid blepharoplasty. However, she decided to wait
for the effect of treatment to disappear before deciding on surgery. |

Case 2
A 52-year-old woman complained of upper lid heaviness, and noticing
excess of skin when applying eye make-up 2 weeks following injection of
botulinum toxin to the upper forehead. She underwent the injection in the
hope that it may prevent the development of future forehead wrinkles. Examination
revealed absent frontalis action with normal eyelid position but with folds
of redundant skin overhanging the skin creases (Figure 3).
Manually elevating the lids removed this overhang, and a diagnosis of latent
dermatochalasis unmasked by botulinum rejuvenation was again made. She
also declined upper lid blepharoplasty. When the frontalis action returned
3 months later there was disappearance of the dermatochalasis (Figure
4). The patient did not undergo further botulinum toxin injection. |

Discussion
Overactivity of facial muscles in actions such as frowning, squinting,
and raising the eyebrows can lead to premature signs of ageing such as
forehead frownlines, glabellar creases, and crow's feet. The onset of these
facial features of ageing can be slowed by the use of botulinum toxin-induced
chemodenervation of the muscles of facial expression.3,4 Botulinum
toxins are toxins produced by Clostridium botulinum. There are seven
serotypes (A-G) of botulinum toxins. Of these,
A is the most potent and was the first to be made commercially available
for therapeutic use. In the UK, botulinum toxin type A (BTX-A) is available
in two forms: Botox (produced by US company Allergan Inc.) and Dysport
(produced by the UK company Ipsen Ltd). The potency of the two products
is not directly comparable unit for unit; as Botox being three to five
times more potent than Dyport.5 The toxin produces a nondepolarising
block at the level of the neuromuscular junction by binding to acetylcholine
receptors of the striated muscle leading to a flaccid paralysis. The onset
of action begins after about 72 h, while the duration of action ranges
between 3 and 8 months with a mean of 4 months. The effect is only temporary
because the muscle generates extrajunctional acetylcholine receptors and
the nerve, in turn, sprouts and reinnervates the muscle.6,7
To date, there have been no long-term adverse effects to the use of BTX-A.
In dermatochalasis, there is redundant skin between the skin crease
of the upper lid and the eyebrows that can lead to a sense of heaviness
in the eyelids, and in severe cases can be cosmetically unsightly and even
impair visual function. Upper lid blepharoplasty is a safe and effective
procedure of treating the condition.8,9 In a subsection of patients,
there is a latent dermatochalsis because of the elevating action of frontalis.
Consequently, loss of frontalis action can make this excess skin become
apparent as a manifest dermatochalsis.
To anticipate the possibility of this occurring is an important part
of the assessment of the patient prior to the use of botulinum toxin for
cosmetic purposes. It is therefore important to assess the effect of frontalis
action on the periorbital regions. By relaxing frontalis and then stabilising
the muscle manually, it is possible to assess whether there is any significant
redundant skin above the lid crease that could lead to a subsequent dermatochalsis
following otherwise successful treatment of forehead wrinkles. If there
is this risk then the patient can be counselled and treated accordingly
either by reducing the dose of the botulinum toxin given or performing
upper lid blepharoplasty if the dermatochalasis fails to improve.
The unmasking of latent dermatochalasis is a potential complication
of the use of BTX-A in the treatment of forehead wrinkles. Thorough preoperative
assessment of these patients can identify those at risk of this problem
and thus reduce the risk of the complication. |
 |
| Acknowledgements
Proprietary/Financial interest:
None. |
 |
| References |
 |
| 1 Carruthers J, Carruthers A.
Treatment of glebellar frown lines with C. botulinum exotoxin. J
Dermatol Surg Oncol 1992; 18: 17-21.
PubMed
2 Carruthers A, Carruthers J.
Clinical indications and injection technique for the cosmetic use of botulinum-A
exotoxin. Dermatol Surg 1998; 24: 1189-1194.
PubMed
3 Foster JA, Wulc AE, Barnhorst
D, Papay F. The use of botulinum A toxin to ameliorate Facial dynamic
lines. Int J Aesthet Reconst Surg 1996; 4: 137-144.
4 Lowe NJ. Botulinum toxin type
A for facial rejuvenation: US and UK perspectives. Dermatol Surg
1998; 24: 1216-1218. PubMed
5 Moore P. Handbook of Botulinum
Toxin Treatment. 1st edn. Blackwell Science Publication: Oxford, 1999.
6 Horn A, Porter JD, Evinger C.
Botulinum toxin paralysis of the orbicularis oculi muscle. Types and time
course of alterations in muscle structure, physiology and lid kinematics.
Exp
Brain Res 1993; 96: 39-53.
PubMed
7 Simpson LL. Kinetic studies
on the interaction between botulinum toxin type A and the cholinergic neuromuscular
junction. J Pharmacol Exp Ther 1980; 212: 16-21.
PubMed
8 Putterman AM, Cosmetic Oculoplastic
Surgery, Vol II, 3rd edn. WB Saunders. Philadelphia: 1999, pp 77-90.
9 Baylis HI, Golberg RA, Kerivan
K, Jacobs J. Analysis and treatment of the ageing face. Dermatol
Clin 1997; 15(4): 635-647. PubMed |
 |
| Figures |
 |
Figure 1
Bilateral dermatochalasis following botulinum injection to the frontalis.

|
Figure 2
Manual elevation of forehead reduces the dermatochalasis.
|
Figure 3
Bilateral dermatochalasis following botulinum injection to the frontalis.

|
Figure 4
Reduced dermatochalasis at 3 months post-injection.

|
 |
 |
 |
 |
 |
Main page
|
|